SPONSORED POST
CHEMSAFE was founded in 2001 by Dr. Antonio Conto and at this day is one of the best independent consultancy companies in Europe for registration of Agrochemicals, Biocides, Chemicals (REACH regulation), Pharmaceutical and FOOD/FEED products.

Antonio has over 30 years of post-graduate experience, mainly in Risk Assessment field and Regulatory Toxicology for chemical, pharmaceutical and food companies. Today his role is mainly focused in managing Chemsafe and reviewing toxicology/safety Expert Reports.
Before founding Chemsafe, Antonio worked as Study Director and Head of C&L Unit in RBM SpA (now Merck-Serono). Antonio is now Member of CHCS (Chemical Hazard Communication Society), SETAC Europe (Society of Environmental Toxicology and Chemistry), ACS (American Chemical Society), SITOX (Italian Society of Toxicology) and SOT (US Society of Toxicology) among others. He has a PHD in Biological Sciences and a Postgraduate Master in Experimental Toxicology. From 2011 he is member of EUROTOX as ERT® European Registered Toxicologist. He is the author of 100 regulatory/scientific papers.
The multidisciplinary team
Along these years Chemsafe has been developed to acquire internal expertise in various fields of activities, which have progressively increased. In 2022, the Company consists in five Business Units, operating in the three main areas of interest: Chemical, Medical and Food/Feed. The five Business Units are managed by Scientists with a solid scientific background and a working experience of at least 10 years.
A Board of Directors (BoD) has been created in 2021.

The BoD task is to help the Managing Director to design the strategic development of the Company over the next years and, at the same time, to manage its growth efficiently, working in strict cooperation with the Heads of the five BUs.
Our multidisciplinary team includes the highest level of knowledge about legal, chemistry, biology, experimental toxicology as well as alternative methods approaches including “in silico”, environmental fate, eco-toxicology, regulatory and scientific networks. All staff at CHEMSAFE is employed full time.
Quality, reliability and competitive prices
Based on an excellent expertise and scientific background, CHEMSAFE manages all projects with quality, reliability and competitive prices. A dedicated Project Leader is appointed to each project.
In 2018, we achieved the QMS (Quality Management System, ISO 9001-2015) certification by Bureau Veritas. The certification was confirmed and expanded to other services in 2021. This achievement was particularly effective for our service to medical area companies that often look for Quality certification.
Our core services are well described in the following picture.
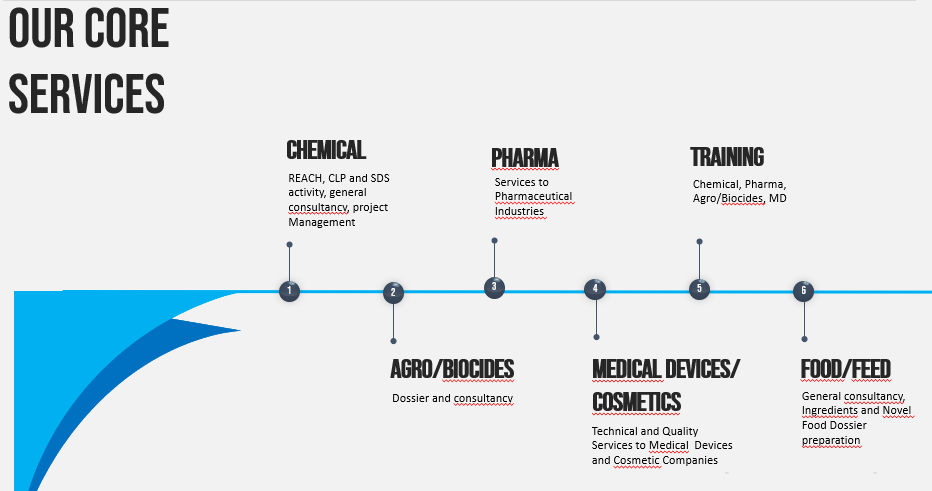
For those who intends to understand better and to have more information we invite to navigate our web site chemsafe-consulting.com.
There is a kind of common background (Fil Rouge) which links all these activities: our expertise and experience in the regulatory toxicology. From the very beginning, we began assessing chemicals for their toxicological properties by searching referenced and selected (by quality) literature sources and/or by testing them on behalf of our customers and providing them with Expert Reports on the toxicological profiles. Step by step this activity evolved, along with evolution of global regulations, toward alternative methods that include the application of the “in silico” methods (Q-SAR or SAR). Similarly and at the same time we developed the risk assessment activities for human health and environmental exposures when required by related regulations and the subsequent dossier preparation.
There are several examples of this type of development within Chemsafe; one of these is the Risk Assessment for Medicinal Products according to EMEA (nowadays EMA) guidelines (2006).
Before the entry into force of these guidelines by the EMEA, we carried out a number of environmental risk assessments for chemicals whose approach was historically regulated by a series of EU Directives and later Regulations. When evaluating the 2006 draft EMEA guidelines we realized that the principles/criteria included in these guidelines were taken from the above-mentioned chemical directives/regulations. It was therefore not so difficult for us to shift this activity from chemicals to medicinal products. Since then, we successfully completed more than 30 ERA (Environmental Risk Assessment) projects including the management of eco-toxicological and environmental fate tests outsourced to third-party laboratories.
Another example, no less important, is the PDE (Permissible Daily Exposure) assessment. When setting a PDE value, the approach is very similar to that used in chemicals regulation to assess the human health risk assessment of chemicals. The trick is to see and apply the “differences” that may be used in different applications and/or different cases. Overall, substance-based toxicological assessment is the commonly used approach.
In the end, the transition from the original more chemical-oriented services to the medical sector as Pharma, Medical Devices and also food/feed was not so difficult. Additionally, the founder’s (Antonio Conto) background in the pre-clinical development was of great help.
As mentioned, these are only two examples of how the company was developed from a scientific/regulatory point of view. On the other side some business decisions were crucial. Mainly the creation of a sales task force in 2017 with BDs dedicated to different areas cooperating directly with the BUs heads.
This has been accompanied by a steady increase of human resources (from 1 to 32 in 20 years).
What’s the future?
Chemsafe is still actively creating and seeking growth opportunities; we need to improve some already established services and develop new ones. As a priority, we would like to focus on internationalisation outside Europe, exploring new market possibilities. Also, why not, to acquire other companies to expand our business in a complementary way.

10010 Quagliuzzo (TO) – Italy
tel. +39 0125 538888
chemsafe@chemsafe-consulting.com


